Vascular Working Group
Summary of goals/project(s) of working group:
The principle role of the SCTC Vascular Working Group (VWG) is to develop improved outcome measures for peripheral vascular manifestations of SSc. The efforts of the SCTC-VWG are being driven by the experiences of our patients. The SCTC-VWG has received international support and has proved a highly effective vehicle for idea generation and consensus building.
Challenges in establishing treatment efficacy in clinical trials of Raynaud’s phenomenon (RP) led the nascent SCTC-VWG to make an appraisal of existing methods for assessing SSc-RP an early priority. The Raynaud’s Condition Score (RCS) is the preferred outcome measure for SSc-RP and included in the provisional core set of outcome measures for SSc clinical trials. The RCS diary collects information on the frequency, duration and severity/impact of RP in SSc and was devised for a negative clinical trial of oral iloprost. The RCS diary is a clinician-derived patient-reported outcome [PRO] instrument and, common to several other SSc-specific PRO instruments, there was no patient involvement in its development. There has been several disappointing controlled clinical trials of promising vasodilator therapies for SSc-RP and there are currently no FDA-approved treatments for the management of SSc-RP. Studies have identified poor agreement between the RCS diary and objective methods for assessing digital microvascular function in SSc. Concerns have also been raised about the magnitude of the placebo effect in clinical trials incorporating the RCS diary.
Progress to date for working group:
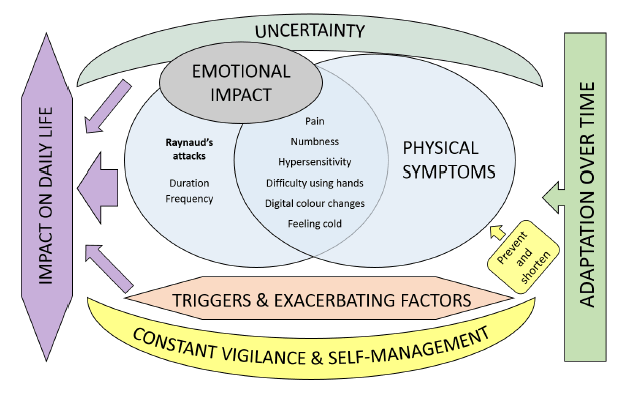
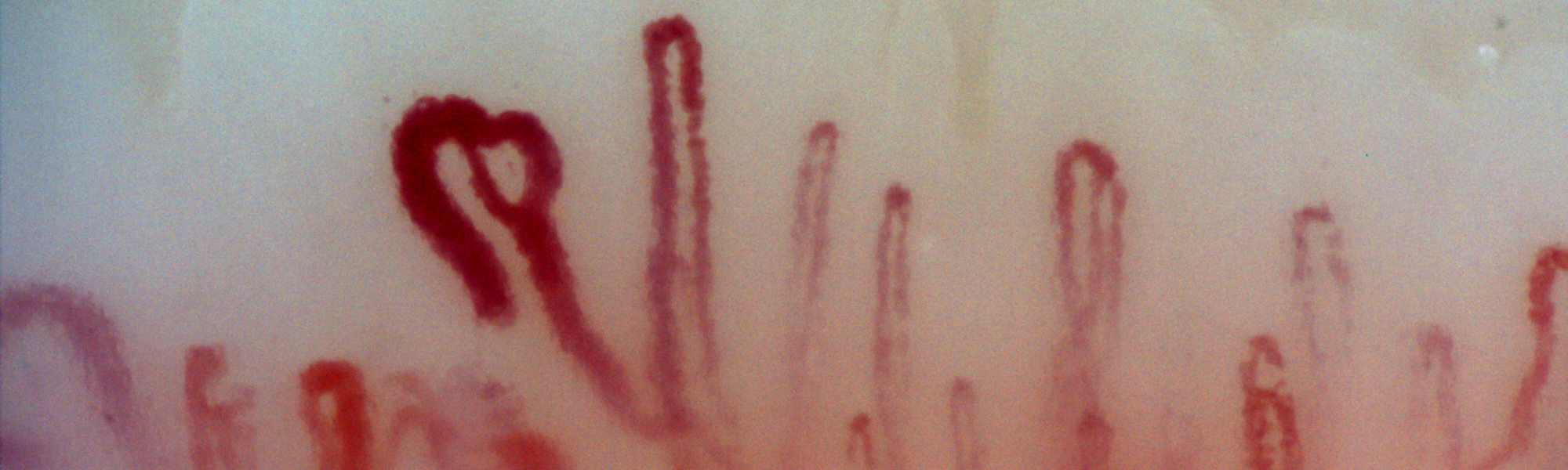
A health professional survey exploring the attitudes of SSc experts towards the RCS diary was undertaken within the SCTC-VWG. The SURPASS (SUbjective Raynaud’s Phenomenon Assessment in Systemic Sclerosis) survey has identified several concerns about the 2-week RCS diary including the respondent burden and an inability to control for factors that might influence the reporting of RP symptoms in SSc [1]. There was strong consensus that limitations of the RCS diary might impede drug development programs in SSc-RP and that a novel PRO for SSc-RP (developed with input from SSc patients and clinicians) was needed [1]. To this end, SCTC funding was obtained in 2016 to devise a conceptual framework for a novel patient-derived PRO instrument for SSc-RP, supported by a steering committee comprising SSc patients, SSc experts and qualitative researchers. The work has closely adhered to regulatory standards concerning the development of PRO instruments. Preliminary work included a comprehensive literature review that has highlighted the significant burden associated with SSc-RP and identified a number of patient experiences not captured by the RCS diary [2]. A multicenter qualitative research study designed to examine the patient experience of SSc-RP from a broad ethnic, geographic and cultural population of SSc patients has supported and expanded these findings[3]. We identified important experiences of SSc-RP not captured by the RCS diary including emotional distress, body image dissatisfaction, impaired social participation and relevant physical symptoms such as feeling “cold”[3]. Our findings have challenged the prevailing paradigm of SSc-RP representing an episodic phenomenon with physical symptoms such as pain, numbness and loss of hand function reported in a more sustained manner and reflecting persistent digital ischaemia. A separate questionnaire-based study has suggested the patient experience of SSc-RP may evolve during the disease course; with late disease being associated with patterns of RP suggestive of more persistent digital ischaemia [4].
The considerable efforts taken by patients to avoid or ameliorate SSc-RP attacks was a major theme of the focus groups [3]. A patient qualitative survey examining patient attitudes towards the RCS diary after completion of 2-week diary collection suggests self-management approaches result in RCS diary returns significantly under-estimating the true burden of SSc-RP [5]. The qualitative survey suggests SSc patients feel the RCS diary is missing important detail on SSc-RP severity [5].
Next steps:
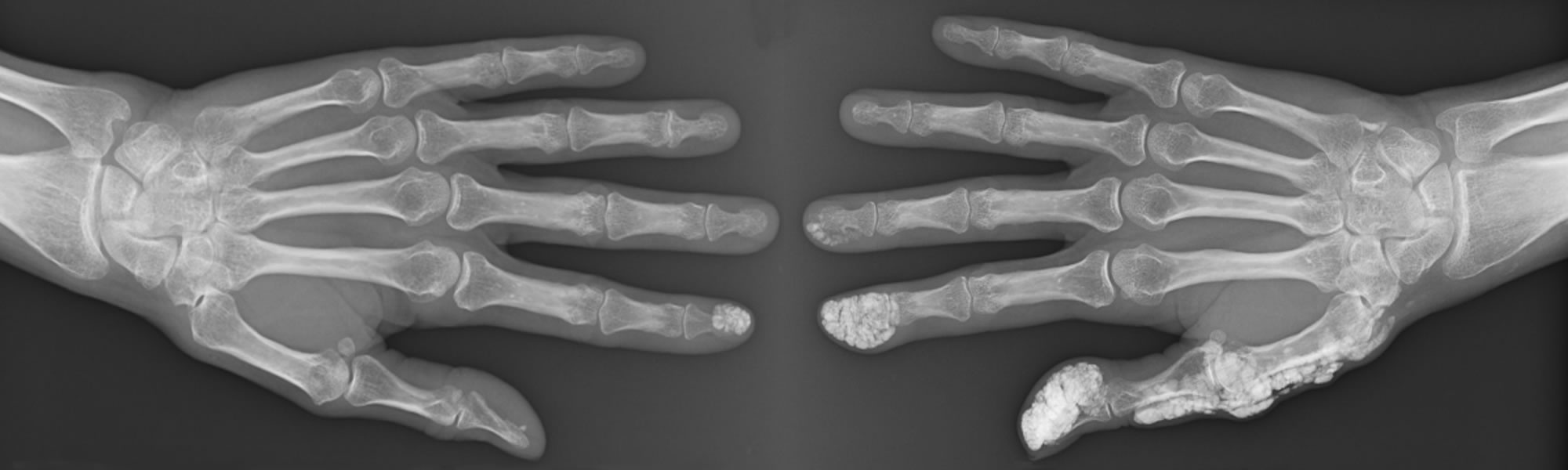
Work within the SCTC-VWG is currently underway on item-generation for a novel PRO instrument for SSc-RP, grounded in the patient experience of SSc-RP identified in our preliminary work. The SCTC-VWG is also supporting new work streams to devise novel clinician and patient-reported assessment tools for digital ulcer disease in SSc (using SCTC funding obtained 2017 – led by M Hughes & J Pauling). We have undertaken a comprehensive literature review [6] and held the first focus group for this parallel programme of work in Bath, UK. We look forward to providing an update of the progress with this work in due course.
What are expected deliverables and timelines for grant/working group?
We hope to commence recruitment to a longitudinal questionnaire-based validation study of the novel PRO instrument for SSc-RP this Winter and undertake a preliminary analysis during Summer 2019. We are looking for English-speaking centres who might be interested in supporting recruitment to this study.
Contact information for how can members get involved:
John Pauling
Royal National Hospital for Rheumatic Diseases (at Royal United Hospitals), Bath, UK
Department of Pharmacy and Pharmacology, University of Bath, Bath, UK
[email protected] or [email protected]
Publications:
-
Patient experiences of digital ulcer development and evolution in systemic sclerosis. Hughes M, Pauling JD, Jones J, Denton CP, Domsic RT, Frech TM, Herrick AL, Khanna D, Matucci-Cerinic M, McKenzie L, Saketkoo LA, Gooberman-Hill R, Moore A.Hughes M, et al. Among authors: pauling jd. Rheumatology (Oxford). 2020 Aug 1;59(8):2156-2158. doi: 10.1093/rheumatology/keaa037.
-
Multicenter Qualitative Study Exploring the Patient Experience of Digital Ulcers in Systemic Sclerosis. Hughes M, Pauling JD, Jones J, Denton CP, Domsic RT, Frech TM, Herrick AL, Khanna D, Matucci-Cerinic M, McKenzie L, Saketkoo LA, Gooberman-Hill R, Moore A.Hughes M, et al. Among authors: Pauling jd. Arthritis Care Res (Hoboken). 2020 May;72(5):723-733. doi: 10.1002/acr.24127.
-
What narrative devices do people with systemic sclerosis use to describe the experience of pain from digital ulcers: a multicentre focus group study at UK scleroderma centres. Jones J, Hughes M, Pauling J, Gooberman-Hill R, Moore AJ.Jones J, et al. Among authors: pauling j. BMJ Open. 2020 Jun 11;10(6):e037568. doi: 10.1136/bmjopen-2020-037568.BMJ Open. 2020. PMID: 32532783
-
Exploring the patient experience of digital ulcers in systemic sclerosis.
Hughes M, Pauling JD.Hughes M, et al. Among authors: Pauling jd. Semin Arthritis Rheum. 2019 Apr;48(5):888-894. doi: 10.1016/j.semarthrit.2018.08.001. Epub 2018 Aug 11.Semin Arthritis Rheum. 2019. PMID: 30205981 -
Pauling, J.D., et al., Patient-reported outcome instruments for assessing Raynaud's phenomenon in systemic sclerosis: A SCTC Vascular Working Group Report. Journal of Scleroderma & Related Diorders, 2018. 3(3): p. 249-252.
- Pauling, J.D., Saketkoo, L.A., Matucci Cerinic, M., Ingegnoli, F., Khanna, D., The patient experience of Raynaud’s phenomenon in systemic sclerosis. Rheumatology, 2018. ePub ahead of print.
- Pauling, J.D., et al., Multi-national qualitative research study exploring the patient experience of Raynaud's phenomenon in systemic sclerosis. Arthritis Care Res (Hoboken), 2018. 70(9): p. 1373-84.
- Pauling, J.D., E.S. Reilly, T., and T. Frech, Evolving symptoms of Raynaud’s phenomenon in systemic sclerosis are associated with physician and patient-reported assessments of disease severity. Arthritis Care & Research, 2018. In press.
- Pauling, J.D., Saketkoo, L.A., Domsic, R.T., Patient perceptions of the Raynaud’s Condition Score diary provide insight into its performance in clinical trials of Raynaud's phenomenon." Arthritis & Rheumatology, 2018. 70(6): p. 973-4.
- Hughes, M. and J.D. Pauling, Exploring the patient experience of digital ulcers in systemic sclerosis. Semin Arthritis Rheum, 2018.
Figure. A conceptual map of the inter-related themes comprising the patient experience of SSc-RP [3].