Gastrointestinal Working Group
Summary of goals/project(s) of working group:
To develop an international collaboration through which we will collect data and determine whether certain microbial genera and/or their products are associated with GI disease activity in SSc. We will also examine the relationship between the participants’ diet, bacterial metabolites, and patient-reported symptoms. These studies will enable us to identify distinct GIT microbiota features of the SSc disease state, understand whether the composition of the microbiome and dietary habits are related to patient symptoms, and identify novel treatment targets to alleviate symptoms in this disease.
Project Details and Logistics:
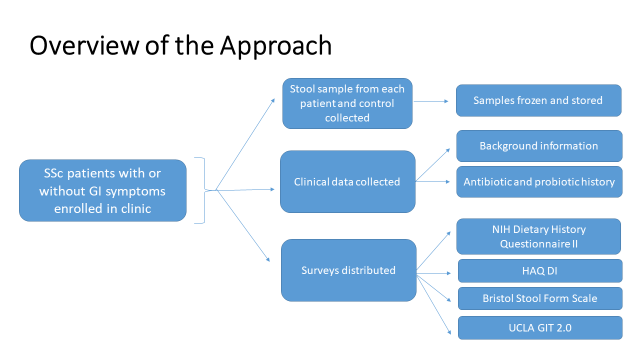
Using a diverse, international population of SSc patients, we will first determine whether GI microbiota compositional differences exist between SSc patients with moderate to severe lower GI dysfunction (High) vs. none-to-mild lower GI dysfunction (Low) (Aim 1). We will then evaluate whether the abundance of specific pathobiont phylotypes are increased, while the abundance of commensal phylotypes are decreased in patients in the High versus Low SSc-GI groups (Aim 2). Finally we will determine whether metabolic alterations in GI microbiota are associated with severity of lower GI dysfunction in SSc patients using shotgun metabolomics (Aim 3).
Participants:
- SSc patients will be recruited consecutively from each site
Inclusion criteria
- Age ≥18 years
- SSc diagnosis (2013 ACR/EULAR criteria)
- Any antibiotics used more than 1 time in a 3-month period over the past year
- Antibiotics used within the 3 weeks immediately prior to stool sample collection
- Diagnosis of inflammatory bowel disease, irritable bowel syndrome or GI malignancy
- Patients with a connective tissue disease other than SSc
Sites who have received IRB approval to conduct this study:
|
Site (Country) |
Principle Investigator(s) |
|
University of California-Los Angeles (USA) |
Elizabeth Volkmann |
|
Johns Hopkins University (USA) |
Zsuzsanna McMahan |
|
University of Ghent (Belgium) |
Vanessa Smith, Karin Melsens |
|
University of Utah (USA) |
Tracy Frech |
|
University of Florence (Italy) |
Silvia Bellando Randone, Marco Matucci-Cerenic |
|
University of Milan (Italy) |
Chiara Bellocchi |
|
Duke-NUS Graduate Medical School (Singapore) |
Andrea Low, Cassandra Hong |
Next steps:
- Complete all material transfer agreements
- Being recruitment and collect clinical data, surveys, and stool samples
What are expected deliverables and timelines for grant/working group?
We plan to apply for funding in the coming year after we obtain more pilot data. An abstract will be submitted for the next ACR annual meeting.
What to get involved? Contact information:
Elizabeth Volkmann: [email protected]
Zsuzsanna McMahan: [email protected]